Tainted medicine: Mass. moves to revoke pharmacists' licenses
Loading...
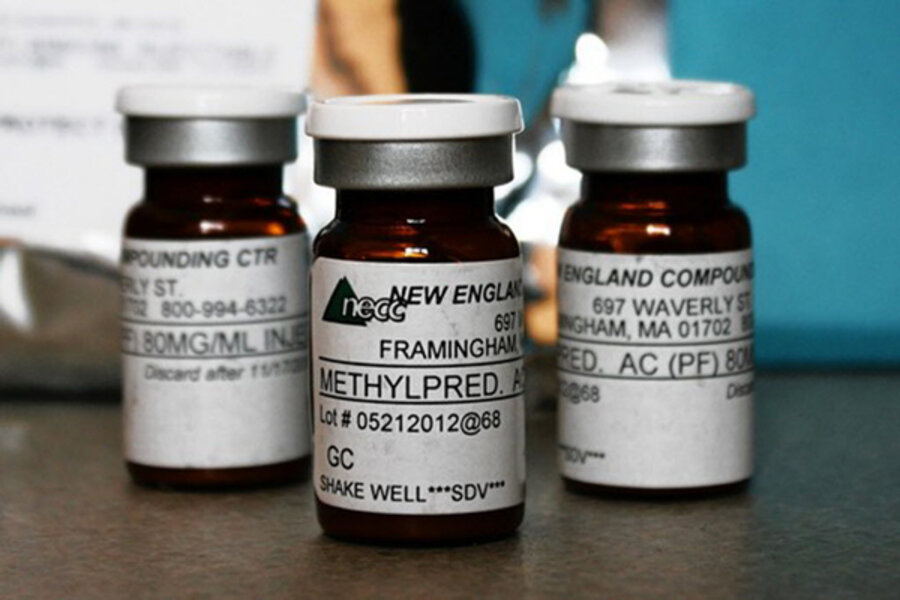
Massachusetts public health officials have moved to permanently pull the licenses of the three top pharmacists at a Bay State compounding pharmacy whose tainted medicine has been linked to more than 300 illnesses and 24 deaths in 17 states.
The move coincided with the release of preliminary findings from state and US Food and Drug Administration inspectors, who say they found evidence that the company, the New England Compounding Center (NECC), violated the terms of its license and fell short of meeting internationally recognized standards for compounding pharmacies that produce sterile drugs. These standards form the basis for state regulations governing compounding pharmacies.
Typically, compounding pharmacies produce relatively small quantities of medicines whose dose or composition is tailored to meet the needs of patients unable to use mass-produced drugs. The pharmacies produce the drugs in response to prescriptions doctors provide.
In NECC's case, the company was making and selling sterile pharmaceuticals in large quantities but without specific prescriptions from an authorized physician or other health-care provider, according to a sampling of preliminary findings the state released on Tuesday.
"NECC was operating beyond the scope of their compounding license, instead acting as a manufacturer," said Madeleine Biondolillo, director of the Massachusetts Department of Public Health's Bureau for Healthcare Safety and Quality, at a press conference outlining the initial findings.
Nationally, 3,000 out of 7,500 compounding pharmacies produce sterile products, according to the International Academy of Compounding Pharmacies. According to Dr. Biondolillo, 25 compounding pharmacies in Massachusetts are qualified to produce sterile drugs.
State and federal investigators found a range of apparent NECC violations beyond mass-production. Among them:
• NECC shipped batches from two lots of the drug in question – an injectable steroid used to relieve pain – before it had results in hand from sterility tests the company performed on product. When the results came back, they indicated no contamination. Still, several sealed vials that came back under the company's recall of the product had black particles floating in them. Investigators are now looking into the thoroughness of the company's tests.
In addition, investigators found that when the company sterilized the steroids, they didn't operate the sterilization equipment long enough to meet minimum standards. The report also notes that the company failed to test the sterilization hardware to make sure it worked properly.
• A boiler next to the pharmacy's clean room had standing water under the boiler, reaching nearby walls. Water is a prime habitat for microbes, including the fungus associated with the outbreak of meningitis pegged to the tainted drug. Investigators have taken samples to see what sort of microbes the water holds.
• Mats designed to collect soil, dust, and other contaminants from the workers' shoes before they enter the clean room were darkened with filth and debris, suggesting the mats weren't changed as frequently as standards require.
• Tabletop boxes known as powder hoods, designed to prevent powders used in formulating a sterile drug from contaminating a product, were not thoroughly cleaned – providing a potential source of contamination.
This is not the first time that NECC has been in trouble with regulators. The company has been the target of special inspections several times over the past 13 years.
"It is tragic that these conditions were allowed to exist," writes Eric Kastago, president and owner of ClinicalIQ, in an e-mail. "There were lots of missed opportunities."
ClinicalIQ is a New Jersey-based consulting firm that focuses on compliance with sterile-compounding standards.
Looking over the particulars in the interim report, many of the problems investigators identified appear to be the result of the "normalization of deviance," a term coined after the space shuttle Challenger accident to describe the way departures from normal procedures become routine when the departures don't lead to consequences.
In NECC's case, many of the items the investigators found appear to be shortcuts the company "took many times before, without consequence," and so they became the new "normal" procedures, suggests Edmund Elder Jr., director of the Zeeh Pharmaceutical Experiment Station at the University of Wisconsin at Madison and a participant in several investigations into compounding-pharmacy practices.
As the probe continues, investigators still are trying to determine how contaminants found their way into the steroid vials. Possible pathways include the raw materials used to make the drugs or additives introduced to ensure any powdered ingredients say dissolved in the liquid after the initial mixing, suggests Stephen Byrn, professor in the college of pharmacy at Purdue University in West Lafayette, Ind.
Since the investigation began Sept. 26, it has expanded to two other pharmaceutical companies affiliated with NECC.
Massachusetts Gov. Deval Patrick announced Monday that the state's pharmacy board would begin immediate, unannounced inspections of compounding pharmacies that produce sterile medicines. It would require yearly reports on the quantities of medicines they produce and the extent of their distribution. Companies would have to report to the state any dealings with federal regulators, such as the FDA.
The Patrick administration also aims to set up a commission to seek out "best practices" among other states' regulators and present recommendations on improvements to Massachusetts' regulatory approach.